Breaking the Wall of Metabolism with 3D and AI
Breaking the Wall of Metabolism with 3D and AI
Global Call 2025 Finalist Interview: Life Sciences
Ramon Sun is a biochemist and molecular biologist specialising in metabolism and spatial biology. He earned his Ph.D. from the Australian National University and completed postdoctoral training at Stanford University. He is currently the Anne and Oscar Lackner Endowed Chair and Associate Professor of Biochemistry and Molecular Biology at the University of Florida College of Medicine. He also founded and directs the UF Center for Advanced Spatial Biomolecule Research (CASBR), a nationally leading facility dedicated to high-resolution spatial metabolomics and imaging mass spectrometry. Sun’s research focuses on how metabolic pathways influence physiology, signalling and disease progression, particularly in the brain and cancer. His lab integrates spatial metabolomics with AI-driven analysis to uncover cell- and tissue-specific mechanisms underlying neurological, neoplastic and age-related disorders.
Which wall does your research or project break?
My research breaks the "Wall of Metabolic Invisibility," revolutionising how we visualise and understand the spatial dynamics of metabolism in health and disease. Traditional metabolomics has been hampered by several key barriers: the inability to integrate diverse biomolecular classes (metabolites, lipids, glycans) due to their differing physical and chemical properties, such as size, solubility and complexity; the limitation of two-dimensional (2D) imaging that fails to capture three-dimensional (3D) tissue architecture; and challenges in sensitive, spatially resolved detection and quantification, which obscure metabolic heterogeneity within tissues.
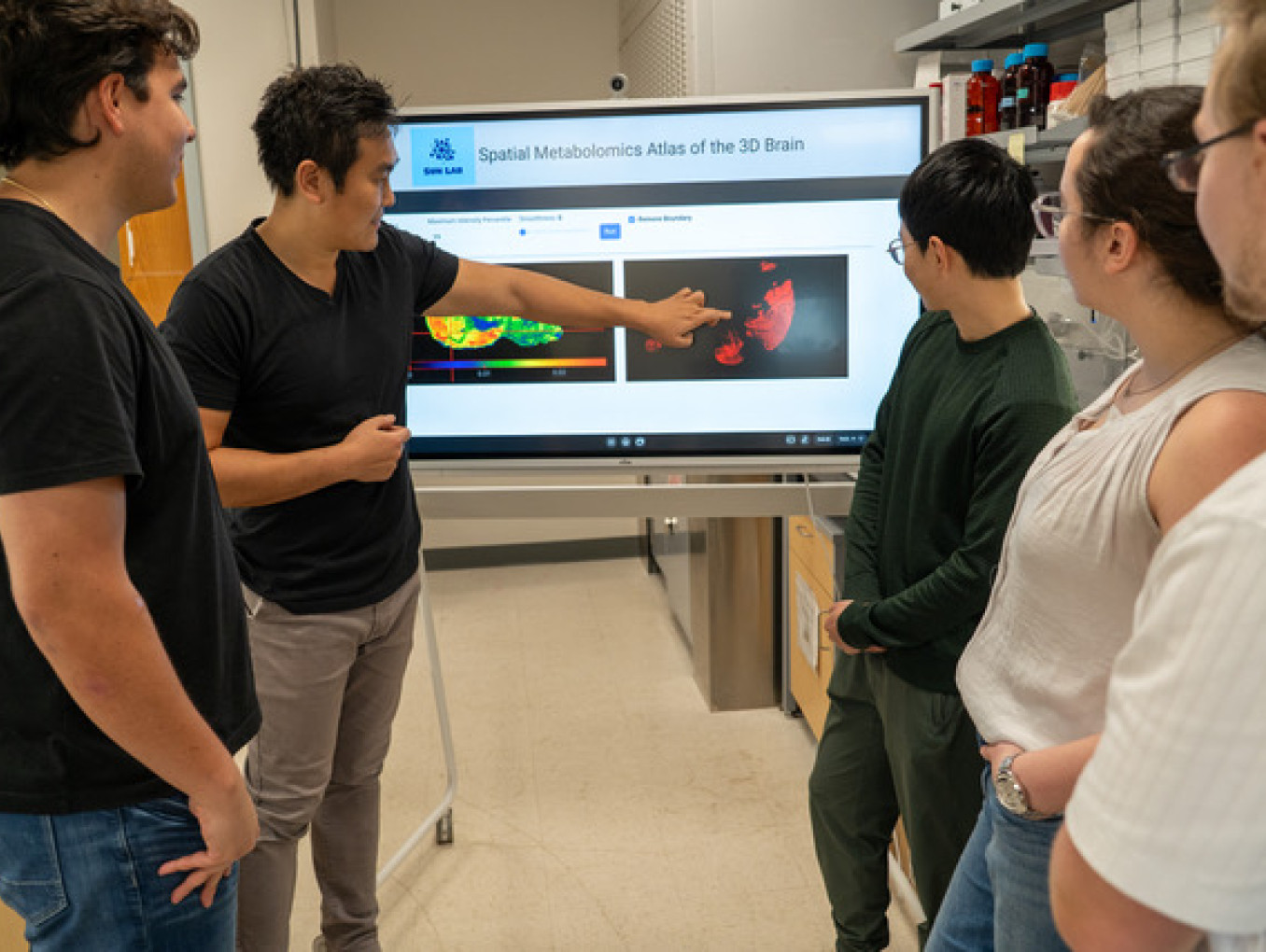
To address these, my work introduces the Spatial Augmented Multionics Interface (Sami), a computational framework that enables simultaneous mapping of the metabolome, lipidome and glycome from a single tissue section using mass spectrometry imaging (MSI). By overcoming analytical incompatibilities, Sami integrates multionics data with spatial coordinates, facilitating high-dimensional clustering, correlation networks and pathway enrichment.
This breaks the wall of fragmented omics silos, providing a unified view of interconnected metabolic landscapes and revealing region-specific demands that were previously invisible. Extending this, I developed MetaVision3D, an AI-driven pipeline that reconstructs 3D metabolic atlases from serial 2D MSI slices. This addresses the "flat" view of tissues by automating precise alignment, interpolation of missing data and smoothing, producing volumetric maps that expose metabolic gradients across complex structures. For instance, it transforms disparate 2D snapshots into cohesive 3D models, enabling deeper insights into spatial metabolic variations.
By linking spatial resolution to metabolic function, our work enables a systems-level view of how molecular metabolism shapes tissue pathology. This breaks through long-standing barriers in metabolism, imaging and organismal biology.
What is the main goal of your research or project?
The goal of my research is to make the invisible world of metabolism visible—both in space and in its biological significance. We aim to understand how metabolism is organised within tissues and how it rewires in disease.
Traditional metabolomics collapses complex spatial signals into bulk averages. It misses how metabolites are distributed across different anatomical and cellular domains. That loss of resolution has limited our ability to generate precise hypotheses about metabolic dysfunction in diseases like Alzheimer’s and cancer.
To address this, we built tools that map metabolism across space, scale and molecular classes. Together, these 2D and 3D spatial tools provide a powerful engine for hypothesis generation. They don’t just describe what’s there—they reveal where to look next and why. They uncover previously unrecognised metabolic patterns and transitions, giving us new leads on mechanisms of disease progression, tissue specialisation and therapeutic vulnerability.
Ultimately, our goal is not just to build better maps—but to reshape the questions we ask in metabolism research. By illuminating metabolic events that were once invisible, our platforms open new directions for the field, especially in understanding how molecules operate within the native structure of tissues. We see this work as laying the foundation for the next decade of metabolism research—where hypotheses are driven not by bulk profiles, but by spatially precise, multi-layered biological insight.
What advice would you give to young scientists or students interested in pursuing a career in research, or to your younger self starting in science?
To young scientists or students embarking on a research career—or to my younger self, wide-eyed and eager at the lab bench—my advice is this:
Be fearlessly passionate and curious. Science isn't just about following established paths; it's about charging into the unknown, where no one has gone before, to tackle the true enigmas that keep the world awake at night.
Embrace that fire in your gut—the one that questions everything and dreams of breakthroughs. It's what propelled me to develop tools like Sami and MetaVision3D, shattering the "Wall of Metabolic Invisibility" by mapping the brain's metabolome in 3D and uncovering its hidden roles in the brain.
Finally, stay passionate about impact. Science isn't abstract—it's about illuminating biology's fundamentals, from tissue homeostasis to disease progression. Go where others hesitate, explore uncharted metabolic landscapes, hypothesise wildly and validate rigorously.
To my younger self: Trust your passion; it will lead you to innovations that redefine fields. Young scientists, the unknown awaits—charge forward fearlessly. Your breakthroughs could rewrite how we understand life itself.